Today, the clinicians who care for those women are getting new interim guidance about the health advantages of instead using the HPV test alone as the primary screen to find cervical cancer or its precursors. Under the new guidance, the Pap smear, which dates back more than 80 years, would still be used for follow-up tests if an HPV test is positive. The Pap smear will still be used for primary screening of women under age 25.
The need for guidance about using the HPV test was triggered last April when the FDA approved one existing HPV test for use in primary cervical cancer screening. Today's guidance, written by a group of cervical cancer screening experts led by University of Alabama at Birmingham gynecologic oncologist Warner Huh, M.D., is being published simultaneously in the journals Gynecologic Oncology, Obstetrics & Gynecology, and the Journal of Lower Genital Tract Disease under the title "Use of Primary High Risk Human Papillomavirus Testing for Cervical Cancer Screening: Interim Clinical Guidance." Also published today in Gynecologic Oncology is the end-of-trial data of the Roche Diagnostics ATHENA HPV trial that enrolled more than 47,000 women in a longitudinal, three-year study of Roche's HPV test.
"Although FDA approval is critically important for introducing a new screening test or algorithm, providers ultimately rely on guidance or guidelines to help them make the best decisions for their patients and want to understand advantages, disadvantages and unknowns associated with a new screening approach," said Huh, who is a senior scientist for the UAB Comprehensive Cancer Center, Director of the UAB Division of Gynecologic Oncology, and is also a board member for both the American Society for Colposcopy and Cervical Pathology and the Society of Gynecologic Oncology.
Major conclusions The two major conclusions of the interim guidance panel are:
• "Because of equivalent or superior effectiveness," the paper says, "primary HPV screening can be considered as an alternative to current U.S. cytology-based (i.e., Pap smears) cervical cancer screening methods."
The authors note that the existing, previously published guidelines still recommend Pap smears alone, or co-testing with a Pap smear and an HPV test, for cervical cancer screening. However, those guidelines from 2011 predate more recent clinical studies of HPV testing that were analyzed in today's paper.
• Women who have a negative HPV test result from their primary screening have a greater reassurance of a very low risk for a future cervical cancer precursor lesion, as compared to women who have a negative Pap smear test in their primary screening. This lower rate of false negative results is a key benefit of the HPV screening. Overall, the panel said, "While there continue to be numerous practical and research questions, primary HPV testing has the potential to further reduce morbidity and mortality of cervical cancer in the U.S.
However, what is most important is that women need to be screened with any strategy, as many women in the U.S. with cervical cancer are either unscreened or underscreened."
"The scientific evidence clearly demonstrates that primary HPV testing outperforms cytology or Pap as a screening test," said Huh. "This has been confirmed from numerous European and Canadian studies as well as the ATHENA trial. There are going to be fewer false negatives with HPV, and arguably, we have been using a less sensitive test for screening for a while now."
Huh added, "Pap smears miss a fair number of adenocarcinomas. "We don't want a test that will miss disease."
From the patient's point of view, the experience of getting an HPV test will be the same as getting a Pap smear. The difference is how the sample is then screened: Instead of a technician looking for abnormal cells (Pap), the HPV sample is put into an automated machine to detect HPV DNA.
Other questions The guidance also addresses four other questions that clinicians may have.
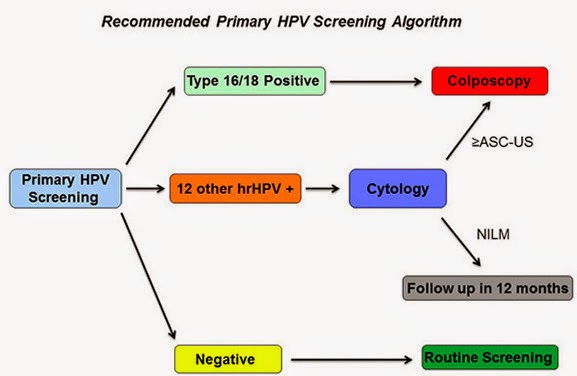
1. How should one manage a positive HPV result? While data are still limited, the study group suggested a flowchart algorithm, as follows. If a woman is positive for HPV genotypes 16 or 18, which convey the greatest risk of developing cervical cancer precursor lesions in the next three years, she should be referred for a colposcopy (an illuminated, magnified examination of the cervix and other genital tissue for premalignant or malignant lesions). If a woman is positive for the 12 other lower-risk HPV genotypes, she should get a Pap smear; and if that Pap smear is also positive, she should then get a colposcopy. If her follow-up Pap smear is negative, she should be retested with another Pap smear in 12 months. This algorithm "achieves a reasonable balance of disease detection with the number of screening tests and colposcopies required to achieve that detection," the panel wrote.
2. What is the optimal interval for primary HPV screening?
Data are limited for determining the optimal screening interval, but the interval should be no sooner than every three years. There is no need to screen more frequently than every three years, since the cumulative occurrence of a cervical cancer precursor lesion called a CIN3+ during the three years after a negative HPV test was less than 1 percent.
3. At what age should one initiate primary HPV screening?
It should not begin before age 25. The study panel noted that about 30 percent of the CIN3+ cervical cancer precursor lesions in the ATHENA study occurred in women ages 25 to 29. A majority of women ages 25 to 29 who have CIN3+ have normal Pap smears. Another 37 percent of the CIN3+ lesions in the ATHENA study were found in women 30 to 39 years old.
The panel did have some concerns that starting at age 25 -- even though it increases detection of disease -- would lead to too many colposcopies in women whose progression to cancer is uncommon.
4. How does primary HPV screening performance compare with co-testing?
The panel said that most of the reassurance of safety provided by a co-test (a Pap smear together with an HPV test) derives from the HPV test. Analysis of about 1 million women screened at Kaiser Permanente Northern California suggests that HPV screening with a three-year interval between negative tests is at least as effective as co-testing every five years. However, co-testing is still an appropriate and recommended screening strategy, Huh noted.
The future As the new advance of primary HPV screening enters into clinical practice, there will be a number of additional questions and concerns, the panel said. First, clinicians need to be aware that false negative tests will still occur -- that is, some women will still develop invasive cancer, even though their HPV tests were negative.
Second, at present there are four commercially available, FDA-approved HPV tests; but only one of them is FDA-approved for primary screening. While the panel hopes that there will be other tests that will be rigorously validated and approved for primary screening sometime in the near future, clinicians should not use a test that lacks a specific primary HPV screening indication.
Third, the panel noted a need for comparative effectiveness studies "that consider projected lifetime number of screening tests, colposcopies and follow-up visits," as well as direct cost comparisons between primary HPV testing vs. Pap smears and co-testing. Further information is also needed about the cancer risks if the interval between HPV tests is extended from three years to five years.
While today's guidance applies to women who receive regular screening for cervical cancer,
the panel also noted the continuing need to identify women who are still unscreened or underscreened.
"One major aspect of cervical cancer prevention that needs to be discussed in light of screening is HPV vaccination," said Huh. "Particularly with the recent FDA approval of the new 9-valent HPV vaccine and evidence that the vaccine decreases HPV and disease prevalence, I have concerns that this will put an additional strain on the performance of cytology (i.e., Pap smear). We will need to look at other tests like HPV as a more appropriate screening test as disease rates decrease over time."